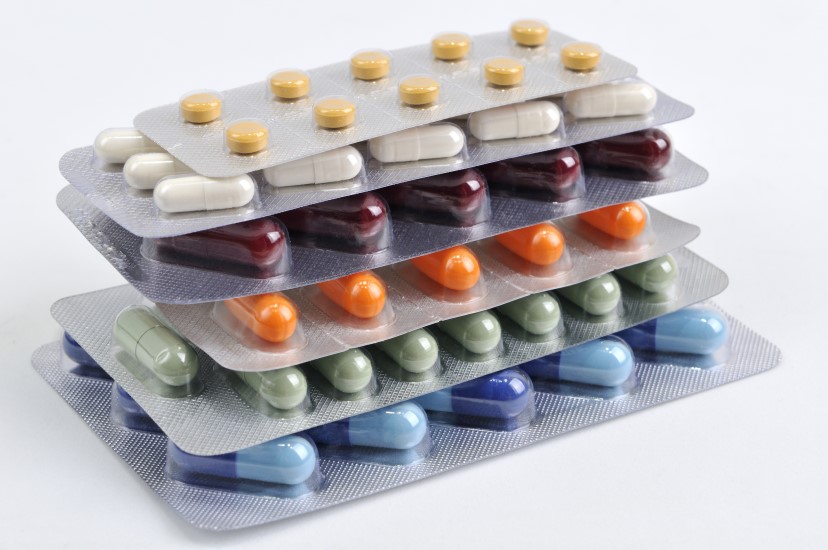
Stricter regulations that will aid in the fight against counterfeit drugs are leading a good number of pharmaceutical companies scrambling to meet the new compliance regulations. In order to do so they are looking at the potential of overhauling not only the packaging, but the distribution and tracking systems in place for all of their manufactured drugs. These regulations are impacting the US, UK, China, Indian, European and even Canadian pharmaceutical manufacturers to name just a few.
Whether the drugs are used in the country of origin or are being shipped out of the country and into the United States, for example, the manufacturers and packaging have to be in compliance with the regulations of the country to which the drugs are being shipped. The World Health Organization recently stated that “up to 10 percent of drugs sold globally are counterfeit” and that points to the need for tighter regulations on packaging.
In order to comply, many pharmaceutical companies are outsourcing the packaging of their drug labeling and packaging to outside sources that are well-versed in the compliance regulations. Compliance, many manufacturers are finding, involves looking for highly-skilled packaging providers; this also involves the distribution process for getting the newly packaged drugs to market.
Griffin Rutgers can help pharmaceutical and/or packaging companies with protecting their products thru its Verinetics product line. The Verinetics concept provides anti- counterfeiting and brand protection solutions for package, serialization, formulation, and document security needs via an extraordinary high-security mark that is unique to each dose or package. The patented Verinetics concept and symbology set consists of shapes that carry manufacturer and individual product specific codes. Implementing secure tracking can help companies with brand security and authentication while tracking their products through the supply chain.
You can contact us by calling 1-800-237-6713 or emailing us at custserv@griffin-rutgers.com. Our team of experts will help you find the right approach for your needs and budget.
Packaging has changed quite a bit over the past few years. Clear packaging, like blister packs and clamshells are becoming a popular choice among manufacturers today. Some studies suggest that shoppers are as much as 400% more likely to pick up a package if they can see the product that is inside.
Growth of international free trade and inadequate drug regulations have led to the expansion of trade in counterfeit drugs worldwide. Technological protection is seen as the best way to avoid this problem. Different technologies came into existence like overt, covert, and track and trace technologies.
The food and medical industry seem to change and grow daily. With that, comes stricter package labeling being required by the FDA. It’s important that your packaging meets the demands of the ever-changing industry and its regulations. At Griffin-Rutgers, we have a solution to meet your labeling requirements.
If you are printing best-before or other fixed or variable information onto sleeves, pouches or cartons, an offline feed and coding system could be just what you need.
Manufacturers and packagers of pharmaceutical products understand that a product identification system for pharmaceutical packaging requires two separate, but highly integrated components. It is mandatory to have a labeling system that can consistently apply labels at the required speed and with a high degree of accuracy. Along with such a labeler packagers need a print...Continue reading→
Pharmaceutical manufacturers and packagers could be facing increased costs due to re-engineering of production lines to satisfy newly upgraded European Union Falsified Medicines Directives (FMD). Printing and packaging systems that carry out coding, and its related post-print inspection, offline as an independent process might make it easier for manufacturers to meet the new compliance requirements...Continue reading→
Stricter packaging regulations that will aid in the fight against counterfeit drugs are leading a good number of pharmaceutical companies scrambling to meet the new compliance regulations. In order to do so they are looking at the potential of overhauling not only the packaging, but the distribution and tracking systems in place for all of...Continue reading→
Brand Owners want to translate their investment in serialization into innovative ways to increase product safety that goes beyond compliance and to yield rich business intelligence data. In order to provide their clients with a solution that achieves both of those goals, Verinetics Corporation and IN2trace Ltd announce a partnership to offer TraxSecur™ Serialized Security™...Continue reading→
TraxSecur™ Serialized Security™ – Now for identifying individual doses Counterfeit, gray market, stolen, or diverted pharmaceutical products would be easier to detect, and those crimes deterred, if legitimate packages and their contents were logically linked with a unique set of security markings. TraxSecur™ was originally developed so that a simple mobile app on a smart...Continue reading→
Accuracy and speed are crucial in all aspects of printing and labeling and this is especially true in the pharmaceutical field. Pharmaceutical packaging and labeling are required to comply with state and federal regulations as well as meeting specific manufacturer and industry requirements. Many pharmaceutical carton and case printing needs can be accomplished with automatic...Continue reading→