Griffin-Rutgers labeling and coding systems are well suited for the pharmaceutical industry. Manufacturers and packagers of pharmaceutical products understand that a product identification system for pharmaceutical packaging requires two separate, but highly integrated components. It is mandatory to have a coding/labeling system that can consistently print in high resolution and apply labels at the required speed with a high degree of accuracy.
Some of these types of packaging can be: pharma blister packs, pharma/medical device cartons, dietary supplements pouches, nutraceutical bottles, along with cannabis tins. Staying current on the regulations in the industry is challenging, but having a printing and labeling system in place that helps adhere to them is key to any operation.
At Griffin-Rutgers we understand the never-ending demand for consistent, accurate labeling, along with the need for print clarity and product tracking mechanisms throughout the packaging and distribution chain. As you consider the purchase, we can help you determine the best product identification (labeling and coding) system for your unique needs. Contact the professionals at Griffin-Rutgers, and we will put our experience to work to select the exact system for you.

Re-Pack Top Panel Labeling System With Bag Feeding Device
Pick & place feeder, conveyor, series 4 stepper driven labeling head
Re-Pack Wrap Labeler (Without Conveyor)
Applicator and wrap station designed to integrate with the customers existing conveyor

Re-Pack Three & Four Side Panel Wrap Labeling System
Line of labeling systems specifically designed for square and rectangular containers

Re-Pack Mini Panel Labeling System
Entry level offering that can be equipped with the majority of the options from our Open Frame Series of labelers
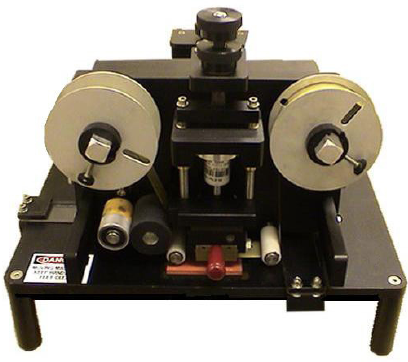
Dalemark 8000TT-HD Table Top Hot Stamp Coder
A heavy duty tabletop hot stamp coder designed to work 24/7 for printing onto flat cartons, envelopes, cards and many other surfaces. Table top systems are designed for operations that are either hand packed or are of lower volumes that don’t need on line coding. They are also often used when several packaging in lines […]
Stricter Package Labeling Being Required By The FDA
The food and medical industry seem to change and grow daily. With that, comes stricter package labeling being required by the FDA. It’s important that your packaging meets the demands of the ever-changing industry and its regulations. At Griffin-Rutgers, we have a solution to meet your labeling requirements.
Coding And Labeling On Blister Packs And Clamshells
Packaging has changed quite a bit over the past few years. Clear packaging, like blister packs and clamshells are becoming a popular choice among manufacturers today. Some studies suggest that shoppers are as much as 400% more likely to pick up a package if they can see the product that is inside.
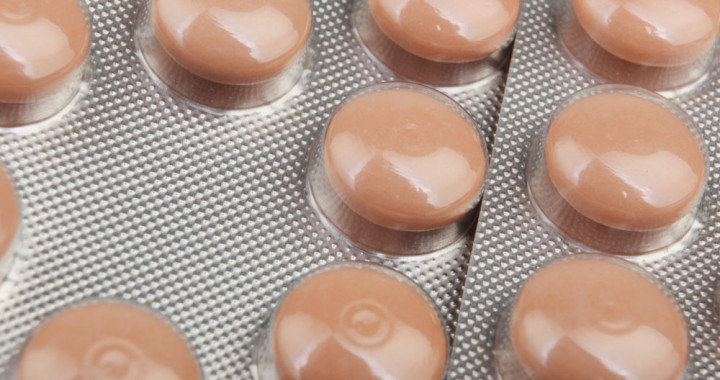
Increased Packaging Regulations Lead To System Overhauls
Stricter regulations that will aid in the fight against counterfeit drugs are leading a good number of pharmaceutical companies scrambling to meet the new compliance regulations. In order to do so they are looking at the potential of overhauling not only the packaging, but the distribution and tracking systems in place for all of their […]
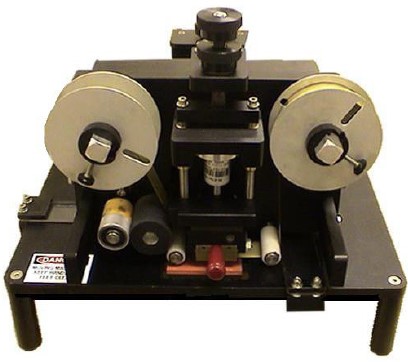
Five Reasons To Choose An Offline Coding System
If you are printing best-before or other fixed or variable information onto sleeves, pouches or cartons, an offline feed and coding system could be just what you need.

Anti-Counterfeit Packaging Coders for Pharmaceuticals
Growth of international free trade and inadequate drug regulations have led to the expansion of trade in counterfeit drugs worldwide. Technological protection is seen as the best way to avoid this problem. Different technologies came into existence like overt, covert, and track and trace technologies.

What Do Pharmaceutical Carton and Case Feeders Do?
Accuracy and speed are crucial in all aspects of printing and labeling and this is especially true in the pharmaceutical field. Pharmaceutical packaging and labeling are required to comply with state and federal regulations as well as meeting specific manufacturer and industry requirements. Many pharmaceutical carton and case printing needs can be accomplished with automatic […]

Labeling and Coding Nuances For The Pharmaceutical Industry
By the time you purchase medicine at the pharmacy, it has gone through a rigorous amount of quality control for both the medicine and its packaging. Manufacturers, when using the proper pharmaceutical coding, labeling and packaging technology can track individual lots of medications throughout the supply chain from point of origin to point of sale. […]

Pharmaceutical Labeling Requires Specialized Printers
Manufacturers and packagers of pharmaceutical products understand that a product identification system for pharmaceutical packaging requires two separate, but highly integrated components. It is mandatory to have a labeling system that can consistently apply labels at the required speed and with a high degree of accuracy. Along with such a labeler packagers need a print […]

Offline Coding Leads To Compliance
Pharmaceutical manufacturers and packagers could be facing increased costs due to re-engineering of production lines to satisfy newly upgraded European Union Falsified Medicines Directives (FMD). Printing and packaging systems that carry out coding, and its related post-print inspection, offline as an independent process might make it easier for manufacturers to meet the new compliance requirements […]







